arabera Palak Bhandari - Jean Augustine SS (2612) 4 years ago
782
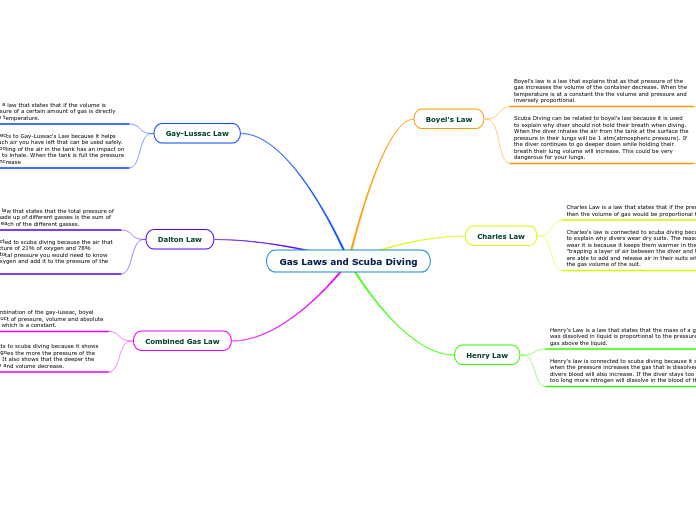
Gas Laws and Scuba Diving
arabera Palak Bhandari - Jean Augustine SS (2612) 4 years ago
782

Honelako gehiago