arabera Lukas Schermerhorn 4 years ago
287
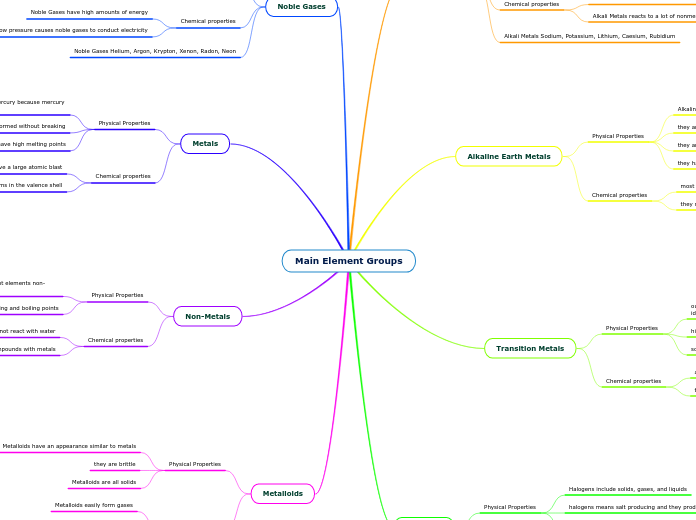
Main Element Groups
Nonmetals, alkali metals, noble gases, metalloids, halogens, and transition metals each display unique physical and chemical properties. Nonmetals are typically non-malleable, non-ductile, and have low melting and boiling points, forming compounds with metals but not reacting with water.