Unit 2: Chemistry (Formula to Name)
prefix shows the number of atoms in the formula. mono = 1, di = 2, tri = 3.
Keeps the name and has a 2 next to it represnting that it has the 2 of the same atoms. ex. H2 = hydrogen
base: starts with the metal and ends with hydroxide. ex. Na(OH) => sodium hydroxide.
binary acid: hydro should be the prefix. then element name, which ends with -ic acid. ex. HBr => hydrobromic acid
oxy acid: polyatomic ending "ate" is removed and replaced with -ic acid. ex. H2CO3 => carbonic acid
is it a polyatomic derivatives?
Ex. Nitrate
2 less oxygen:
hyponitrite (NO)1-
1 less oxygen:
nitrite (NO2)1-
Normal:
chlorate (ClO3)1-
1 more oxygen:
perchlorate (NO4)1-
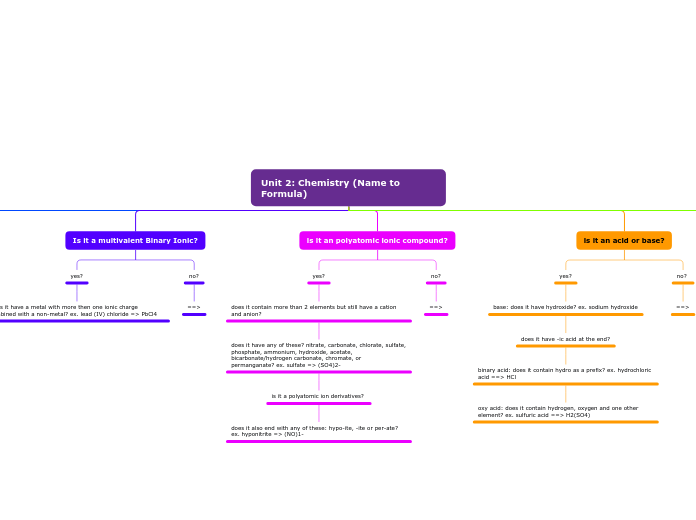
Is it a polyatomic ionic compound?
yes?
Cation (+) followed by anion, only changing to "ide" if single atom anion. ex. Au2O3 => gold (III) oxide
is it binary compound?
Yes?
metal's name comes first. nonmetal comes in second. non-metal ending is changed to "ide"
is it a multivalent?
yes? Then the name of the metal goes first. Afterwards, add Roman numerals in brackets. the non-metal ends with -ide. ex. tin (II) oxide
Unit 2: Chemistry (Name to Formula)
is it a molecular compound?
does any of the elements have: mono, di, tri, tetra, penta, hexa, hept, octa, nona, deca? ex. phosphorus pentasulphide => PS5
is it a diatomic?
is it written as: (naturally have 2 of the same atoms) hydrogen - h2, nitrogen - N2, chlorine - Cl2, iodine - I2, oxygen - O2, fluorine - F2, bromine - Br2
is it an acid or base?
no?
base: does it have hydroxide? ex. sodium hydroxide
does it have -ic acid at the end?
binary acid: does it contain hydro as a prefix? ex. hydrochloric acid ==> HCl
oxy acid: does it contain hydrogen, oxygen and one other element? ex. sulfuric acid ==> H2(SO4)
is it an polyatomic ionic compound?
does it contain more than 2 elements but still have a cation and anion?
does it have any of these? nitrate, carbonate, chlorate, sulfate, phosphate, ammonium, hydroxide, acetate, bicarbonate/hydrogen carbonate, chromate, or permanganate? ex. sulfate => (SO4)2-
is it a polyatomic ion derivatives?
does it also end with any of these: hypo-ite, -ite or per-ate? ex. hyponitrite => (NO)1-
Is it a multivalent Binary Ionic?
Does it have a metal with more then one ionic charge combined with a non-metal? ex. lead (IV) chloride => PbCl4
Is it a binary compound?
no?
==>
yes?
Does it have metal as 1st and non-metal as 2nd? ex. lithium (metal) and oxygen (non-metal) ==> Li2O