by Loh Ang 12 years ago
388
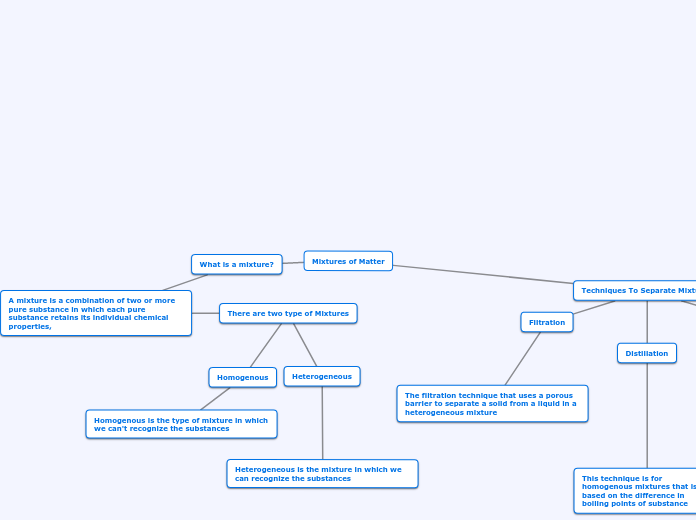
Seperation Techniques
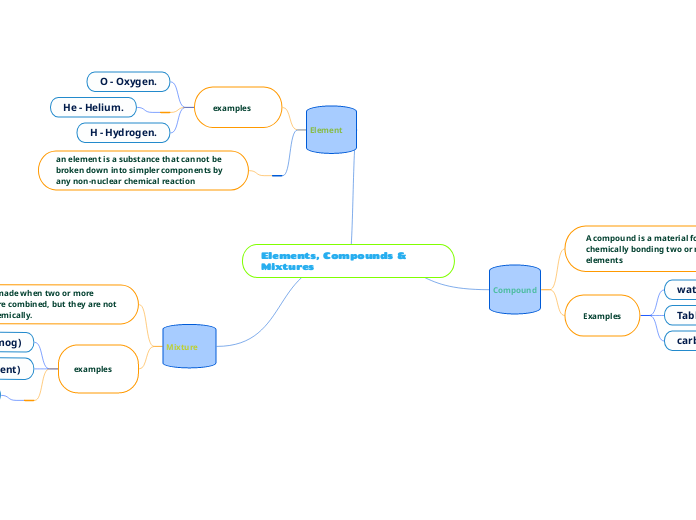
Several techniques are employed to separate mixtures based on their distinct physical properties. Chromatography is a versatile analytical method used to isolate colored components within a mixture, with applications like detecting food additives, RNA fingerprinting in forensics, and identifying poisons or drugs.